Which Is Most Likely to Form a Negative Ion
In electron attachment ionization negative ions are formed by electron attachment in the gas. Which is most likely to form a negative ion.
Required fields are marked.

. An ion is formed when a neutral atom looses or gains electrons. Correct answer to the question Which is most likely to form a negative ion. Thus a nitrogen atom will form an anion with three more electrons than protons and a charge of 3.
Thus a nitrogen atom will form an anion with three more electrons than protons and a charge of 3. Cations are the positive. Ba Ca d Si Р O O C1.
An element with atoms that have eight valence electrons. Aluminum a member of the IIIA family loses three electrons to. TEND to form negative ions ie.
When an atom loses a valence electron it becomes an _____ ion. Nonmetals form negative ions anions. An element from group 17.
Chlorine In the given elements chlorine 287 is a non metal with highest electronegativity. How a cation is formed. In the given elements chlorine 287 is a non metal with highest electronegativity.
Which elements will likely form a negative ion. An element from group 17 B. The elements that are most likely to form negative ions are the halogens.
Are ionic compound negative. And thus nitrogen oxygen fluorine chlorine etc. Which element is MOST likely to form an ion with a negative 2 charge.
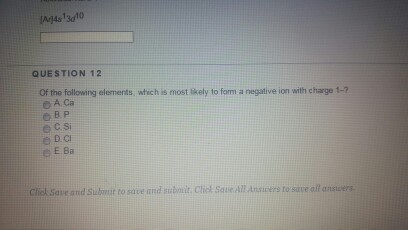
If more electrons are present the ion is negative and referred to. A nitrogen atom must gain three electrons to have the same number of electrons as an atom of the following noble gas neon. Of the following elements which is most likely to form a negative ion with charge 1.
An ion is an atom or group of atoms that has an electric charge. An element from group 1 D. Why does aluminum form a 3 ion.
The element which is most likely to form negative ion is fluorine. This is the best answer based on feedback and ratings. Which element is most likely to form a negative ion with a charge of.
Halogens have 7 valence electrons and will readily acquire an 8th to form an anion. The attraction between oppositely charged ions is. Hence it is most likely to form a negative ion with charge 1.
Which is most likely to form a negative ion A an element from group 17 B a metal C an element from Group 1 D an element with atoms that have eight valence electrons. Iron silver nickel whilst most other nonmetals typically form anions eg. The noble gases helium neon argon krypton xenon and.
If more protons are present the ion is positive and is known as a cation. An element from group 17. When an atom looses electrons it results in the formation of positive ion known as cationWhen an atom gains electrons it results in the formation of negative ion known as anion.
Which of the following is the correct name for MgCl2. Your email address will not be published. Chemistry questions and answers.
Ba Ca d Si Р O O C1. Yet like all metals aluminum is capable of forming an ion by losing electronsin this case three. Clearly the PARENT atoms or molecules are oxidizing species.
Nonmetals form negative ions anions. Of the following elements which is most likely to form a negative ion with charge 1. N3 O2 F and Cl.
Which atom is most likely to form a negative ion. 100 2 ratings d O is the Answer. Hence it is most likely to form a negative ion with charge 1.
Aluminum a member of the IIIA family loses three electrons to form a 3 cation. Ions are formed when the number of protons in an atom does not equal the number of electrons. A nitrogen atom must gain three electrons to have the same number of electrons as an atom of the following noble gas neon.
Leave a Reply Cancel reply. Of the following elements which is most likely to form a negative ion with a -2 charge. Do Group 7 elements form negative ions.
Which is most likely to form a negative ion. An element from group 1. Ionic compounds contain positively and negatively charged ions in a ratio that results in an overall charge of zero.
Ions that are made up of more than one atom are called Polyatomic Adams Which is most likely to form a negative ion an element from group 17 a metal an element from Group 1 or an element with atoms that have 8 valence electrons. Which is most likely to form a negative ion. Most other metals form cations eg.
Given the fact that every proton has a positive charge and that most atoms tend to be neutral in charge this means that there are usually 13 electrons with a negative charge present in an atom of aluminum.

Ionic Bonding Biology Definition Role Expii
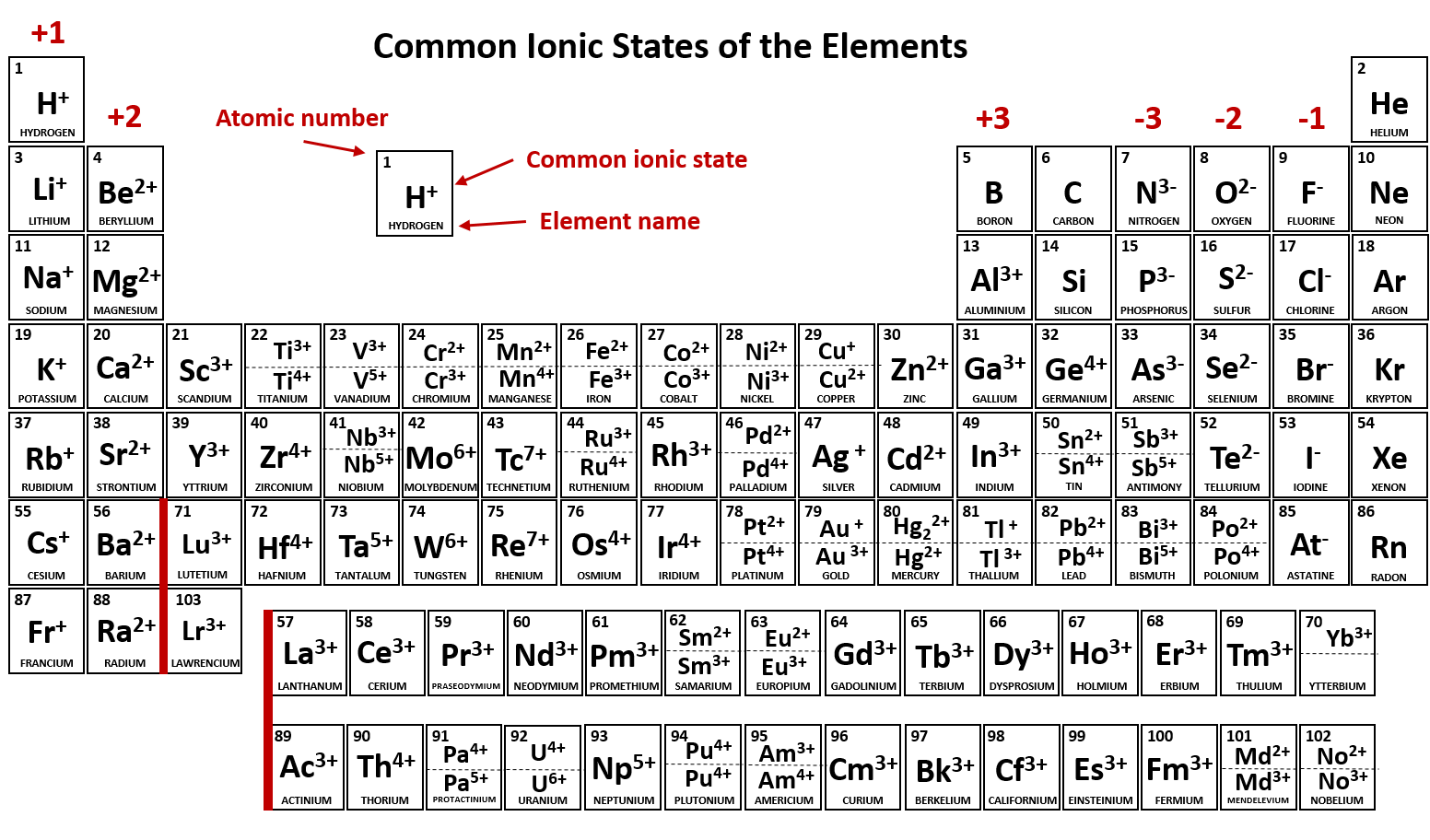
Ch104 Chapter 3 Ions And Ionic Compounds Chemistry

Solved How Many Unpaired Electrons Are There In A Ca Ion Chegg Com
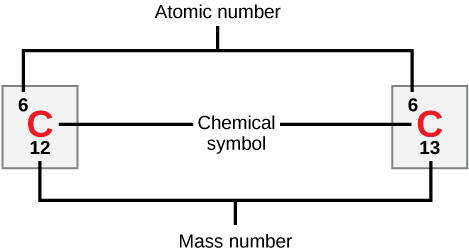
Atoms Isotopes Ions And Molecules The Building Blocks Biology 2e
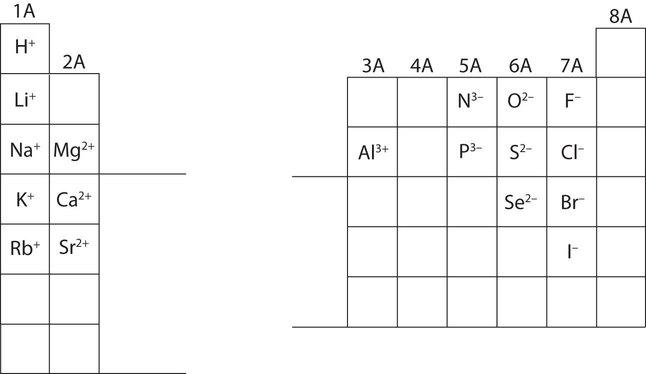
4 7 Ions Losing And Gaining Electrons Chemistry Libretexts

Electron Affinity Of The Elements

Solved E Nlissu 7 Of The Following Elements Which Is Most Chegg Com

Chemistry Ch 8 Flashcards Quizlet

Carbon Group Element Chemical Elements Britannica

Which Is Most Likely To Form A Negative Ion Plz Help Me Brainly Com
Ionization Energy And Electron Affinity
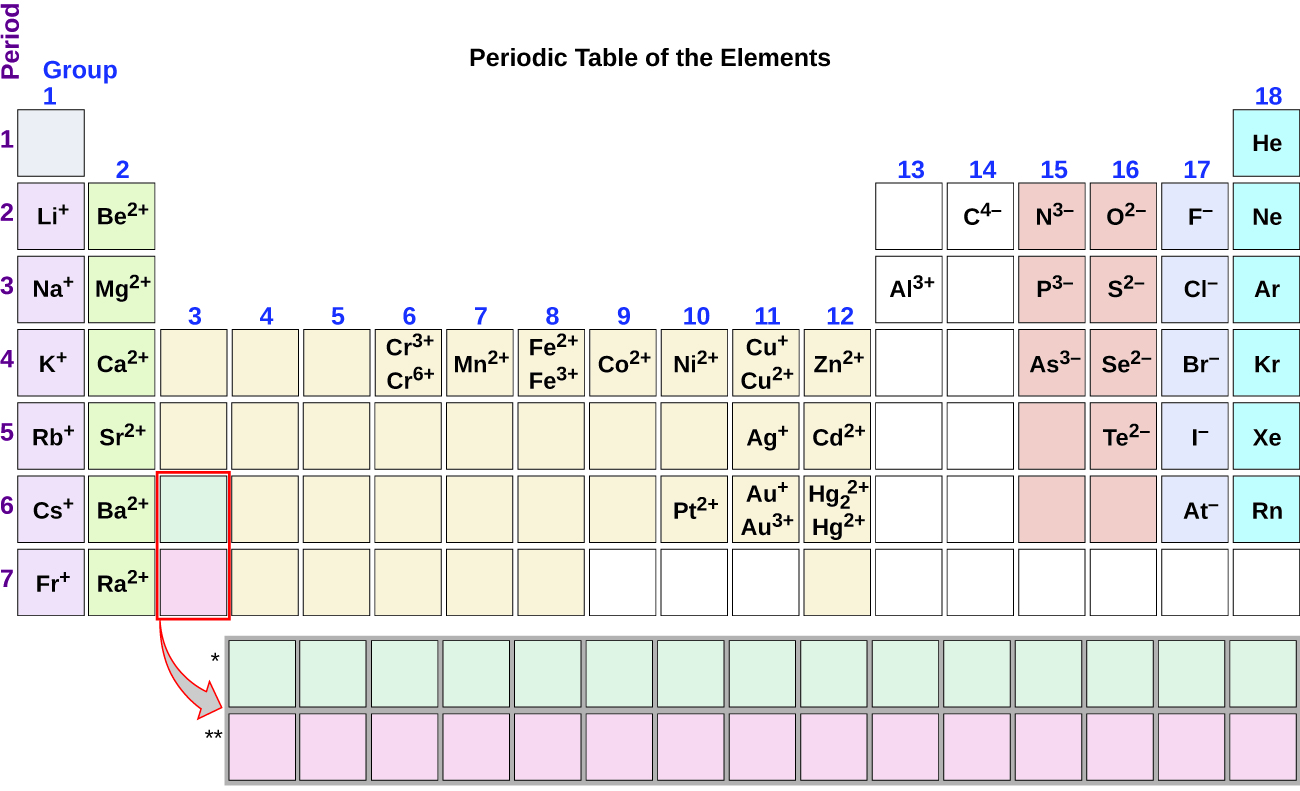
2 6 Molecular And Ionic Compounds Chemistry

Solved A Cao S B Nacl S C Sro S D Csbr S E Kbr Chegg Com

Elements On The Left Side Of The Periodic Table Will Most Likely Form

Take Five 1 Ppt Video Online Download
Ch103 Chapter 4 Ions And Ionic Compounds Chemistry
Solved Iag4s 13010 Question 12 Of The Following Elements Chegg Com


Comments
Post a Comment