Can You Change the Number of Neutrons in an Atom
If you change the number of neutrons in an atom you create A a cation B an anion If you change the number of neutrons in an atom you School University Of Connecticut. This means there are 6 neutrons approximately.
4 When you change number of electrons on an you produce a an.
. Carbon would still be carbon but you would have changed its atomic mass. The addition of a. If you change the number of neutrons in an atom you get an isotope of the same element.
For example Carbons atomic numbernumber of protons is 6 and the mass number is 12011. You cant change the mass number of an atom because the mass number is the number of protons which is the atoms atomic number I hope this helped Changing the number of protons in an atom would. The subatomic particle that never changes is the electron.
What particle in an atom never changes. If you change the number of neutrons in an atom you get an isotope of the same element. A An atom is a solid mass of material.
The atomic number from the atomic mass will give you the calculated number of neutrons in the atom. If you change the number of electrons somehow then there will be less negative charge. Atomic mass however can change and we call these isotopes.
If you change the number of neutrons in an atom you create _____. What happens if you change the number of neutrons in an element. Therefore an elements atomic number will never change.
The atomic number describes the number of protons. An anion carries a negative charge and has more electrons than protons. Since the vast majority of an atoms mass is made up of its protons and neutrons subtracting the number of protons ie.
B The particles that form an atom are equidistant from each other. If there are many atoms of an element that are isotopes the average atomic mass for that element will change. 5 Which equation below would you use to determine the number of neutrons in a given nucleus.
This is due to the same number of valence electrons and therefore bonding and hybridisation will be the same. 6 When you change the number of electrons the mass number changes. It becomes negatively or positively charged.
Hope this can help. When you change the number of electrons on an atom you produce a different. An example could be a carbon 14 atom decaying into carbon 12 atom.
Subtract the atomic number from the atomic mass. The addition of a neutron can make an atom radioactive. We consider electrons to have virtually no mass so we can ignore them.
If you change the number of neutrons you wouldnt change the element of the atom. Therefore you can subtract the atomic number from the mass number to find the number of neutrons. The addition of a neutron can make an atom radioactive.
If you change the number of protons in an atom somehow then there will be less positive charge. Do note that you cannot endlessly add neutrons to an atom. 3 What must change in an atom for it to become a new element.
If you change the number of neutrons in an atom you will also change its charge element. If you change the number of neutrons in an atom you get an isotope of the same element. A a cation B an anion C an isotope D a different element.
Why can the number of neutrons never change. If you change the number of neutrons in an atom you get an isotope of the same element. A isotope b element c ion d charge8.
The number of neutrons or electrons in an atom can change without changing the identity of the element. The change of number of neutrons does not affect the charge of the atom. 442018 Printable Chemistry Quiz - Atom Basics b ion c.
Which of the following is the best description of an atoms physical structure. Changing the number of neutrons of an atom changes its. Neutron numbers are able to change the mass of atoms because they weigh about as much as a proton and electron together.
An elements atomic number will never change that is because the atomic number is its identity. If the atomic mass reaches its upper limit then the decaying of the isotope will occur very quickly. An atom that has the same number of protons atomic number as other atoms of the same element but has a different number of neutrons.
The atomic number is the number of protons. When you change the number of protons in an atom you will change the atom from one element to. The number of neutrons in an atom can change but the term for the amount of neutrons is called an isotope.
The number of protons and neutrons added together is. If you change the number of neutrons somehow nothing will happen because it carrys no charge at all. The number of protons of an atom cannot change via any chemical reaction so you add or subtract electrons to get the correct charge.
Meanwhile the chemical properties of the atom will not change. All it will affect is your average atomic mass which is the sum of protons and neutrons. The numbers after the decimal point represent the usually very small mass of the.
2 When you change the number of you change the element. The addition of a neutron can make an atom radioactive. Does changing the number of neutrons change the element.
When you change the number of protons in an atom you will change the atom from one element to a different element. O number d isotope. The Atomic Number will change.
When you change the number of protons in an atom you will change the atom from one element to a different element. Lets Answer The World. The atomic mass is the sum of the protons and neutrons in the nucleus in an isotope you have more neutrons than you have protons making the mass larger than the stable nuclei.
Neutrons do not have a net electric charge so the number of neutrons does not matter in the calculation. Now you would take the Elektronik Mass minus the atomic number in order to get the neutrons.

Daniel S Model The Electrons That Are In The Atom Are Green Apples The Protons Are Green Peas And The Neutrons Are The Red Apples My Chose Was Nitrogen And I

Isotope Atoms With The Same Number Of Protons Atomic Number But Different Atomic Masses

Atomic Structure Pixel Art Self Checking Activity Free Science Worksheets Covalent Bonding Worksheet Scientific Method Worksheet

The Atom The Smallest Unit Of An Element Protons And Neutrons In Nucleus Surrounded By One Or Shells Of Electrons Neutral Atoms Protons Electrons Ppt Download

Atomic Mass Worksheet Chemistry Worksheets Mass Number Chemistry

This Is A Free 130 Slide Powerpoint Quiz Game About Atoms Nucleus Protons Neutrons Electrons Atomic Number Fun Classroom Activities Science Classroom Atom
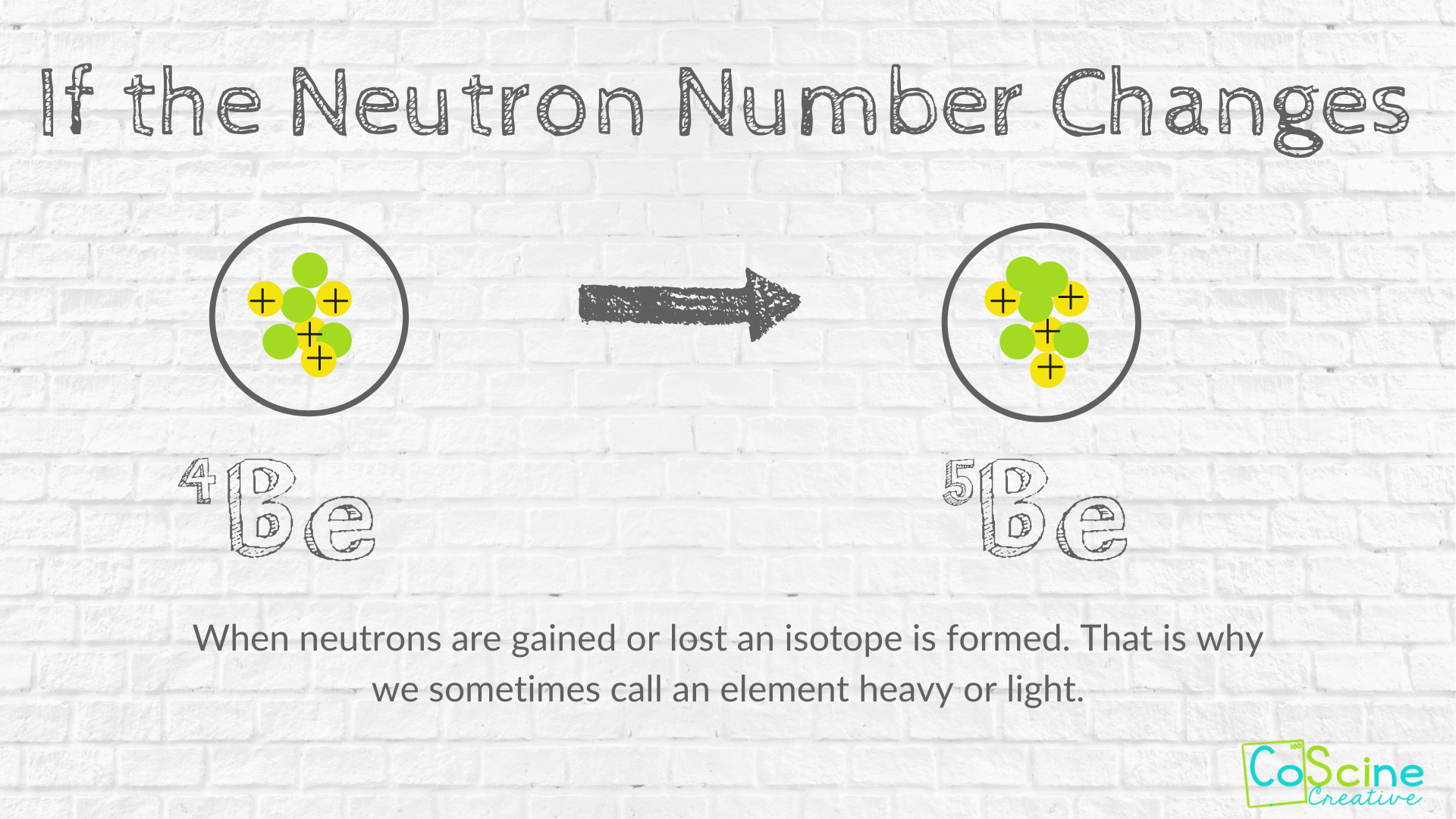
How Does Changing Numbers Of Subatomic Particles Effect The Atom Coscine Creative
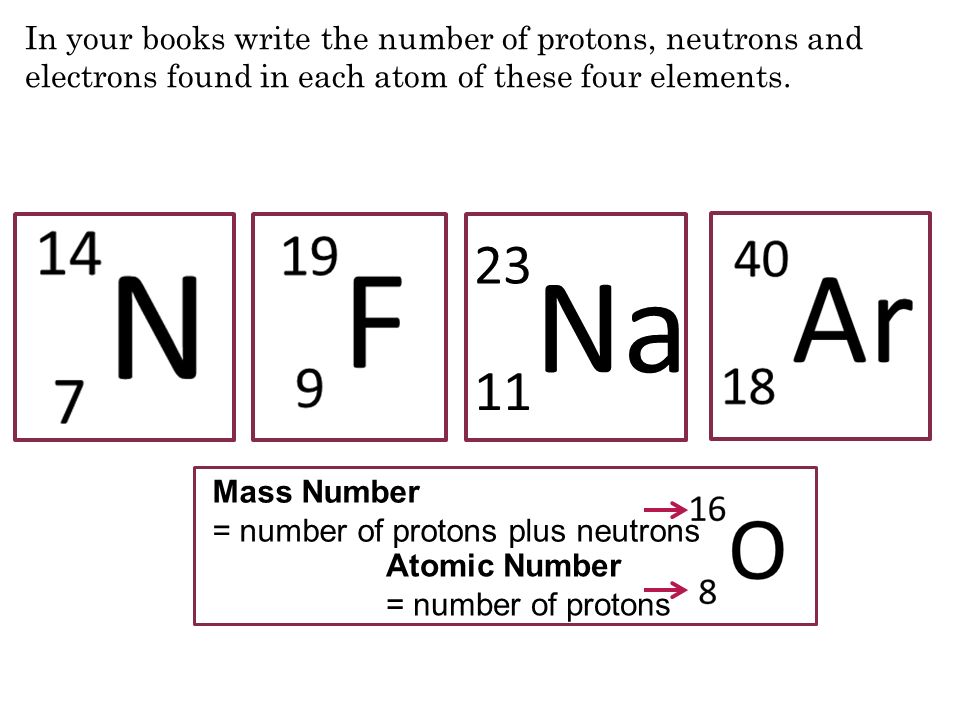
In Your Books Write The Number Of Protons Neutrons And Electrons Found In Each Atom Of These Four Elements Mass Number Number Of Protons Plus Neutrons Ppt Download

What Is Radiocarbon Dating Earthsky Org 8th Grade Science Science Chemistry Class Notes
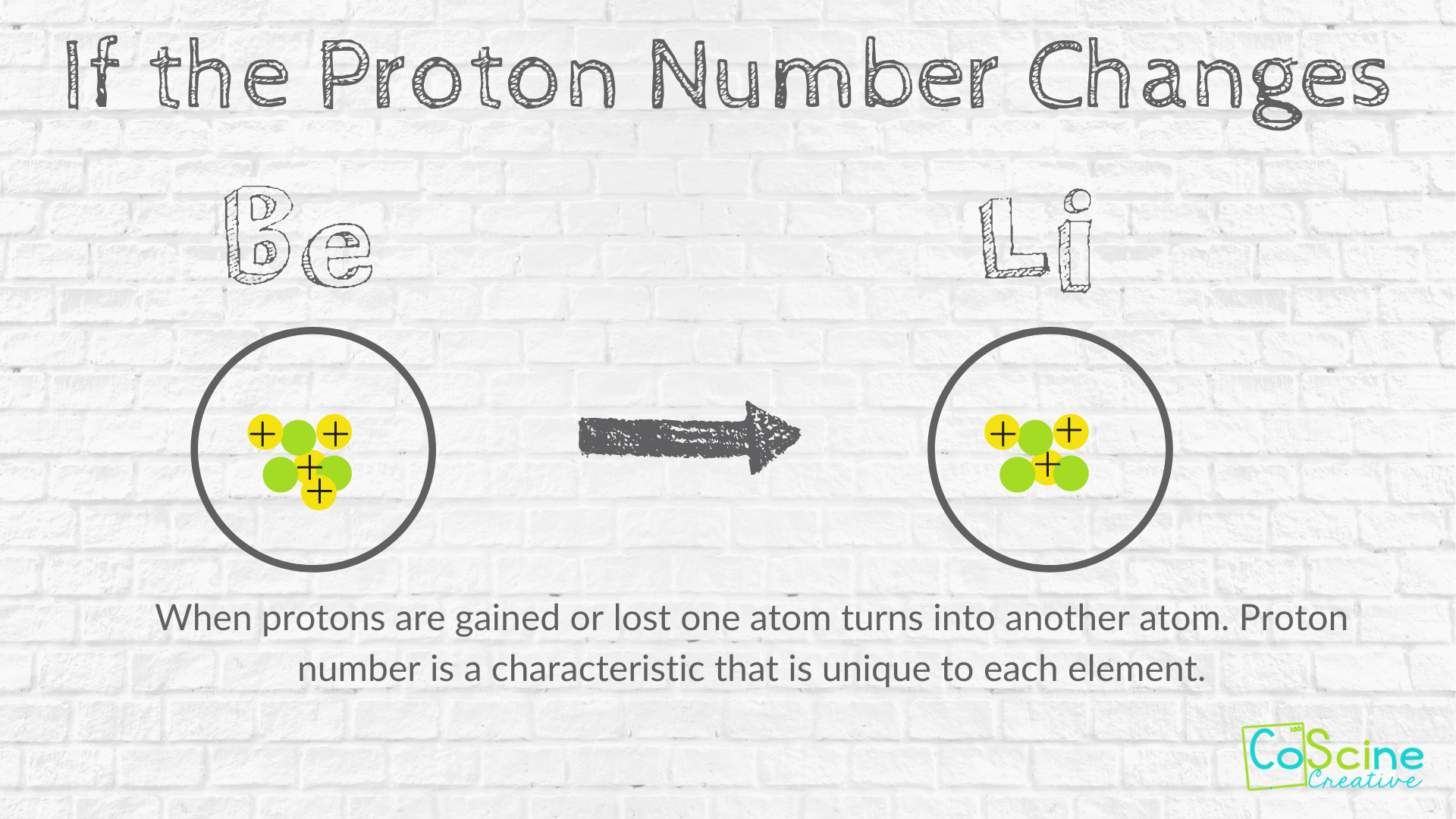
How Does Changing Numbers Of Subatomic Particles Effect The Atom Coscine Creative

Isotope Easy Science Science Student Science Facts Science Memes

Atom And Ion Builder Learning Resources Interactive Protons

Carbon Atom On White Background Structure This Vector Diagram Shows The Proton Sponsored White Background Shows C Carbon Atom Model Atom Atom Model
Discovery Education Physical Science Series Atomic Structure And The Periodic Table

Definition Of Mass Number With Examples Isoptopes Mass Number Definition Of Mass Atomic Mass Unit

Super Teacher Worksheets Chemistry Worksheets Relationship Worksheets

How Does Changing Numbers Of Subatomic Particles Effect The Atom Coscine Creative

Calculating Parts Of An Atom Practice Worksheet 2 Practices Worksheets Worksheets Chemistry Worksheets


Comments
Post a Comment